|
Stripe smut, caused by the fungus Ustilago striiformis,
and flag smut, caused by Urocystis agropyri, are two
leaf or foliar smuts widely distributed in the world and destructive
to many turfgrass species. Smut fungi weaken the grass host making
the plant easy to kill when under severe stress. Plants infected
with flag smut are killed more readily than are plants infected
with stripe smut. Flag smut is often more prevalent in early spring,
while stripe smut generally predominates in late spring and early
autumn. These smut fungi grow systemically throughout a grass plant,
and both smut fungi may infect the same grass plant at the same
time and even the same leaf. Once infected, a plant remains so for
life. Infected plants are often weakened and invaded by other organisms.
Leaf smuts, together with high temperatures and drought, cause
grass plants to exhibit stunted growth, a brown to blackish brown
appearance, a general decline, and early death. Dead patches of
grass often appear in heavily infected turf during midsummer; weed
invasion soon follows (Figure 1).
The stripe and flag smut fungi infect about 100 species of turf
and forage grasses, both cultivated and wild (Table 1). A number
of highly specialized varieties and pathogenic races of these fungi
are restricted to certain cultivars and species of grass. The diseases
occur most commonly on annual bluegrass and Kentucky bluegrass (especially
on the cultivars Delta, Geronimo, Merion, Newport, Park, Rugby,
and Windsor). Creeping bentgrass, colonial bentgrass, redtop, fescues,
common timothy, several wild-ryes and wheatgrasses, orchardgrass,
perennial or English ryegrass, and quackgrass are also commonly
infected. Leaf smuts are favored in locations having excess thatch,
frequent irrigations or rains during spring and summer, turf that
is 3 years or more old, a pH below 6.0, and where susceptible grass
cultivars are grown.
Back to Top
|

Figure
1.
Severe stripe smut in Kentucky bluegrass lawn.

Figure
2. Dark
streaks in Kentucky bluegrass leaves due to leaf smut infection.
|
Symptoms
Due to the killing of individual plants and the distinct upright
growth of infected plants, the turf, in an overall view, appears
clumpy and patchy. Smutted plants are most noticeable during cool
weather in the spring and autumn, appearing pale green to slightly
yellow or brown, stunted, and more upright than healthy plants.
Single plants, or irregular patches up to one foot or more in diameter
may be affected.Short to long, narrow, and yellow-green streaks
(sori) develop between the veins in infected leaves and leaf sheaths.
These streaks soon become silvery to dull gray and extend the entire
length of the leaf blade and sheath (Figure 2). The grass epidermis
covering the streaks soon ruptures, exposing blackish brown, dusty
masses of smut spores (teliospores). After dispersal of the spores,
the leaves soon split and shred into ribbons, turn brown, curl from
the tip downward, turn light brown and die. In addition, leaves
of infected bluegrass and bentgrass plants tend to remain stiff
and erect rather than lax and spreading. The symptoms are usually
most evident in middle to late spring and autumn, when temperatures
average 50 to 65 F (10 to 18 C). Affected plants do not tiller as
profusely or produce as many rhizomes or stolons as healthy plants,
nor do they develop as extensive a root system.
Smutted plants are often difficult to find during hot, dry weather
because a large percentage of such plants often die during summer
droughts (Figure 1). Both smut fungi decrease leaf turgor and water
potentials of infected plants under drought stress. Once infected,
grass plants will rarely, if ever, recover unless properly treated
with a systemic fungicide.
Under close mowing, both smut fungi produce identical symptoms.
Positive diagnosis can be made only through a microscopic examination
of the teliospores produced by the smut fungus. The spores of Ustilago
striiformis are single cells, round to elliptical in shape
and covered with prominent spines (Figure 3). The spores of Urocystis
agropyri are smooth and roundish and are composed of one
to four, dark reddish brown, fertile cells (teliospores) surrounded
by several smaller, empty, or sterile cells (Figure 4) forming a
spore ball.
|
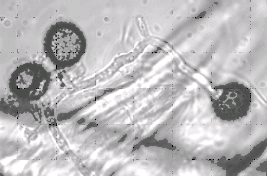
Figure
3.
Three stripe smut spores germinating in drop of water. (Dr. C.F.
Hodges)
|
Disease Cycle
|
The stripe and flag smut fungi overwinter and oversummer as dormant
mycelium in the meristematic tissue of crowns and nodes of infected
plants and as dormant teliospores in or on grass debris, living
plants, and soil. The teliospores are carried to the thatch and
soil by many agents, including wind, rain, shoes, mowing, watering,
raking, dethatching, coring, and other turf maintenance practices.
Spores may also be transported on the seed. These teliospores may
lie dormant in soil for up to 3 years (4 years on stored seed) before
they germinate.
When suitable conditions occur in the spring and autumn, a teliospore
germinates to produce mycelium on which minute spores (sporidia)
may be borne, although this is apparently rare on certain grasses,
such as Kentucky bluegrass and creeping bentgrass. Each sporidium
then germinates and forms a germ tube. When the germ tubes of opposite
mating types fuse (conjugate), an infection hypha forms that penetrates
susceptible host tissue directly. Invasion may occur through the
coleoptile of seedling plants and actively growing (meristematic)
tissues produced by the lateral or auxiliary buds on the crowns,
rhizomes, and stolons of older plants that come into contact with
germinating spores. Once inside the grass plant, smut hyphae develop
systemically in the direction of plant growth, with new leaves,
tillers, rhizomes, and stolons becoming infected as they form. The
mycelium continues to grow within developing tissues.
|
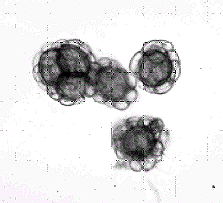
Figure
4. Four spores of flag smut fungus (Urocystis agropyri). (Dr.
C. F. Hodges)
|
Teliospore formation begins with thick, tangled mats of mycelium within
infected grass tissues. The mycelium then breaks up to form masses of
blackish brown, greasy teliospores that are released when the host tissues
rupture, shred, and die.
Smutted plants in new turfgrass areas are uncommon, indicating limited
infection of seedling plants from soil- or seedborne teliospores. The
large number of diseased grass plants in turf areas more than 3 years
old is probably caused by the invasion of lateral buds and the growth
of smut fungi from perennially infected crowns. Once infected, watering
and high fertility, which stimulate plant growth during droughts, create
conditions that favor the buildup of leaf smuts. Such practices keep the
systemically infected grass plants from dying during hot, dry weather.
Back to Top
Control
1. Grow a blend of several cultivars that generally show resistance to
leaf smuts and other major diseases. Bluegrass and bentgrass cultivars
apparently differ greatly in their resistance to leaf smuts. Because numerous
races of the two smut fungi exist, it is difficult to predict the relative
resistance or susceptibility of a cultivar in any given location. Kentucky
bluegrass cultivars showing good resistance to one or both leaf smuts
include A-20, A-34 (Bensun), Adelphi, America, Aquila, Baron, Birka, Bonnieblue,
Bristol, Brunswick, Campina, Challenger, Champaign, Cheri, Columbia, Delft,
Eclipse, Enita, Enmundi, Entoper, Fylking, Glade, Majestic, Merit, Midnight,
Monopoly, Nugget, Parade, Plush, Ram I and II, Sydsport, Touchdown, Vantage,
Victa, and Wabash (see Table 2). Kentucky bluegrass cultivars rated as
susceptible to very susceptible include Galaxy, Geronimo, Merion, Park,
Pennstar, Ruby, and Windsor.
Stripe smut has been reported to infect the following creeping bentgrass
cultivars: Arlington, Cohansey, Congressional, Evansville, Old Orchard,
Penncross, Penneagle, Pennlu, Seaside, Toronto, and Washington. The races
of the stripe smut fungus that attack Kentucky bluegrass do not
infect creeping bentgrass.
2. Sow only seed that is not surface contaminated with teliospores
and is treated with a captan- or thiram-containing fungicide, or start
with disease-free sod, sprigs, or plugs of a resistant cultivar. During
hot weather, the smut fungi become dormant in bentgrass stolons and the
healthy-appearing stolons continue to grow. When cooler weather returns
in the autumn, the smut fungus resumes growth and symptoms reappear in
the stolons.
3. Remove thatch in early spring or late summer when it has accumulated
to 1/2 inch. Use a "vertical mower," "power rake,"
"aerifier," or similar equipment. These machines can be rented
at many garden supply and tool rental stores.
4. Avoid frequent light irrigations during spring and summer. During
summer or early fall droughts, water established turf thoroughly early
in the day so the grass can dry before dusk. Water infrequently and deeply,
moistening the soil at each watering to a depth of 6 inches or more.
5. Apply nitrogen-containing fertilizers sparingly during the summer
months (no more than 1/2 lb of nitrogen per 1000 sq ft per month).
6. Renovate and overseed infected turf with a blend or mixture of relatively
resistant cultivars (Table 2).
7. Where practical, remove the clippings when smutted leaves are evident.
8. Yearly treatment with a systemic fungicide is expensive, but it checks
the pathogen(s). Fungicides that are suggested for the control of leaf
smuts are given in the current edition of University of Illinois Urban
Pest Control Management Guide. Two applications of a systemic fungicide
are needed in late fall with the second application 14 to 21 days later
just before winter dormancy occurs. Apply the fungicide at the recommended
rate per 1000 sq ft in 5 to 10 gal of water. Immediately after each treatment,
drench the fungicide into the soil, applying the equivalent of an inch
of water (600 gal per 1000 sq ft) to make sure that the fungicide moves
down into the root zone. The manufacturer's directions should be followed
carefully. Use the lower fungicide rates in preventive programs, higher
rates in curative programs. Only one of the fungicides listed in the Management
guide need be used. Fungicide use and restrictions are subject to change
without notice. Always read and follow the current package label instructions
and precautions.
Table 1. Some Turf, Forage and Wild Grasses Reported
to be Susceptible to One or More Physiologic Varieties and Races of Stripe
Smut (Ustilago striiformis), Flag Smut (Urocystis agropyri),
or Both
GRASSES SUSCEPTIBLE TO BOTH STRIPE AND FLAG SMUT
|
X Agrohordeum macounii = Elymus macounii
quackgrass or couchgrass (Agropyron repens)
beardless or bluebunch wheatgrass (A. spicatum = A.
inerme)
bearded couch or slender wheatgrass (A. trachycaulum)
cheatgrass (A. sp.)
browntop or colonial bentgrass (Agrostis capillaris
= A. tenuis)
creeping bentgrass (A. stolonifera = A. alba,
A. palustris)
alpine foxtail (Alopecurus alpinus)
fringe brome (Bromus ciliatus)
awnless or smooth brome (B. inermis = B. pumpellianus)
bluejoint (Calamagrostis canadensis)
orchardgrass or cock's-foot (Dactylis glomerata)
Canada wild-rye (Elymus canadensis = E. wiegandii)
blue wild-rye (E. glaucus) |
western wheatgrass (E. smithii = Elytrigia
smithii, Agropyron occidentale, A. smithii,
A. spicatum var. molle)
beardless wild-rye (E. triticoides)
Virginia wild-rye (E. virginicus = E. striatus)
bluebunch or Idaho fescue (Festuca idahoensis)
foxtail or squirrel-tail barley (Hordeum jubatum)
June grass (Koeleria nitida = K. cristata)
Leucopoa kingii = Festuca kingii
alpine cat's or alpine timothy (Phleum alpinum)
common timothy (Phleum pratense)
bulbous bluegrass (Poa bulbosa)
Canby bluegrass (P. canbyi)
Kentucky bluegrass (P. pratensis)
Sandberg bluegrass (P. sandbergii, P. secunda)
Sitanion jubatum
spike trisetum (Trisetum spicatum) |
GRASSES SUSCEPTIBLE ONLY TO STRIPE SMUT
|
|
fairway crested wheatgrass (Agropyron cristatum)
thick-spike wheatgrass (A. dasystachyum)
Agrostis castellana
spike redtop (A. exarata)
redtop (A. gigantea)
A. humilis
A. hyemalis
A. microphylla
autumn or brown bentgrass (A. perennans)
Ross redtop (A. rossae)
rough bentgrass (A. scabra = A. geminata)
European beachgrass (Ammophila arenaria)
mountain hairgrass (Deschampsis atropurpurea)
tufted hairgrass (D. caespitosa)
Elymus semicostatus subsp. Striatus
= Agropyron striatum
Siberian wild-rye (E. Sibiricus)
|
American beachgrass (A. breviligulata)
broom-sedge (Andropogon virginicus)
vernalgrass (Anthoxanthum sp.)
Arctagrostis latifolia
oatgrass (Arrhenatherum sp.)
Avena pubescens
American sloughgrass (Beckmannia syzigachne)
quaking-grass (Briza sp.)
plains reedgrass (Calamagrostis montanensis)
C. pickeringii
Scribner reedgrass (C. scribneri)
timber oatgrass (Danthonia intermedia)
|
nodding fescue (Festuca obtusa)
western fescue (F. occidentalis)
sheep fescue (F. ovina = F. brachyphylla,
F. ovina var. brachyphylla)
English bluegrass or meadow fescue (F. pratensis = F.
latior)
Thurber fescue (F. thurberi)
sweetgrass (Hierochloe sp.)
velvetgrass or Yorkshire-fog (Holcus lanatus = Notholcus
anatus)
bottlebrush (Hystrix patula = Elymus hystrix)
Chinese wild-rice or false wheatgrass (Leymus chinensis =
Elymus chinensis, E. pseudoagropyrum)
English or perennial ryegrass (Lolium perenne)
purple oniongrass (Melica spectabilis)
muhly (Muhlenbergia sp.)
reed canarygrass (Phalaris arundinacea) |
alpine bluegrass (Poa alpina)
annual bluegrass (P. annua)
arctic bluegrass (P. arctica)
Chapman bluegrass (P. chapmaniana)
Canada bluegrass (P. compressa)
P. curtifolia
alkali bluegrass (P. juncifolia)
P. longifolia
Nevada bluegrass (P. nevadensis)
fowl or roughstalk bluegrass (P. palustris = P.
serotina)
P. reflexa
roughstalk bluegrass (P. trivialis)
Nuttall alkaligrass (Puccinellia nuttalliana)
Sesleria sp.
squirreltail (Sitanion hystrix = Elymus elymoides) |
GRASSES SUSCEPTIBLE ONLY TO FLAG SMUT
|
|
goatgrass (Aeglilops sp.)
Baker wheatgrass (Agropyron bakeri)
bearded wheatgrass (A. X subsecundum var. andinum)
thin grass (Agrostis diegoensis)
beardgrass (Andropogon sp.)
oatgrass (Arrhenatherum sp.)
California or mountain brome (Bromus carinatus = B.
aleutensis)
hairgrass (Deschampsia sp.)
Elymus aristatus
basin or giant wild-rye (E. cinereus = E. condensatus)
E. scabrus = Agropyron scabrum
Elytrigia sp.
chewings or red fescue (Festuca rubra = F. rubra
subsp. rubra, F. rubra var. lanuginosa,
F. rubra var. prolifera)
fowl mannagrass (Glyceria striata = G. nervata)
Hierochloe sp.
Hordeum brachyantherum = H. boreale
ryegrass (Lolium sp.)
California melic (Melica imperfecta)
big bluegrass (Poa ampla)
Wheeler bluegrass (P. nervosa = P. wheeleri)
bread or common wheat (Triticum aestivum = T.
sativum,
T. vulgare, T. timopheevii)
|
American dunegrass (E. mollis)
E. riparius
|
Table 2. Modern Kentucky Bluegrass Cultivars Adapted
to Illinois and Reported to be Moderately to Highly Resistant (R)a to
one or More Diseases
|
Kentucky Bluegrass Cultivars
|
"Helminthosporium"
diseases
|
Leaf Smuts
|
Leaf and stem rust
|
Summer patch & necrotic
ringspot
|
Sclerotinia dollar spot
|
Typhula blight
|
Septoria leaf spot
|
Red thread
|
|
A-20
|
R
|
R
|
R
|
R
|
R
|
|
(b)
|
R
|
|
A-34 (Bensun)
|
R
|
R
|
R
|
R
|
|
|
|
|
|
Adelphi
|
R
|
R
|
R
|
R
|
R
|
R
|
R
|
R
|
|
Baron
|
R
|
R
|
R
|
R
|
R
|
R
|
|
R
|
|
Bonnieblue
|
R
|
R
|
R
|
R
|
R
|
R
|
|
R
|
|
Brunswick
|
R
|
R
|
R
|
R
|
R
|
|
|
|
|
Cheri
|
R
|
R
|
R
|
R
|
R
|
|
R
|
|
|
Enmundi
|
R
|
|
R
|
R
|
R
|
|
|
R
|
|
Enoble
|
R
|
|
|
|
|
|
|
|
|
Fylking
|
R
|
R
|
R
|
|
|
|
R
|
|
|
Geronimo
|
R
|
|
|
|
R
|
|
|
R
|
|
Glade
|
|
R
|
R
|
R
|
|
R
|
|
|
|
Majestic
|
R
|
R
|
R
|
R
|
R
|
|
R
|
R
|
|
Monopoly
|
R
|
R
|
|
R
|
|
R
|
|
R
|
|
Nugget
|
R
|
R
|
R
|
|
|
R
|
R
|
R
|
|
Parade
|
R
|
R
|
R
|
R
|
R
|
|
R
|
|
|
Plush
|
R
|
R
|
R
|
|
R
|
|
|
R
|
|
Rugby
|
R
|
R
|
R
|
R
|
R
|
|
|
|
|
Sydsport
|
R
|
R
|
R
|
|
R
|
|
R
|
|
|
Touchdown
|
R
|
R
|
|
R
|
|
|
R
|
R
|
|
Vantage
|
|
R
|
|
R
|
R
|
|
|
|
|
Victa
|
R
|
R
|
R
|
R
|
|
|
|
R
|
a A resistant (R) rating does not mean that a particular cultivar will
be resistant in all locations every year. Due to the presence of physiological
races or strains of the various fungi that cause these diseases, a cultivar
may be susceptible in one locality and highly resistant in another. This
is especially true of powdery mildew and is the reason we omitted this
disease from our ratings.
b A blank under a given disease does not necessarily indicate susceptibility.
In some cases it means that no data are available on which to evaluate
the relative susceptibility or resistance to a particular disease.
Back to Top
|



