Reports on Plant Diseases |
RPD No. 664 - Phytophthora Root Rot and Dieback
of Rhodondendrons and Azaleas in the Midwest
|
March 1988
|
[ Symptoms ] [ Disease Cycle
] [ Control ]
|
Species of Rhododendron, which include azaleas as well as rhododendrons,
are colorful flowering shrubs that are beautiful additions to any landscape.
Unfortunately, these plants are attacked by two very widespread and serious
disease complexes: root rot and dieback.
Rhododendrons grow best in a location protected from excessive wind and
sun, in a porous, well-drained soil with high-moisture-holding capacity
(preferably with an abundance of organic matter) with an acid reaction
(pH 4.5 to 5.5); and with an organic mulch of good insulating quality
covering the soil surface.
|

Figure 1.
Rhododendron dieback caused by Phytophthora cinnamomi.
|
Root rot or Rhododendron wilt (Figures 1, 2, and 3) in the Midwest is caused
by the soiborne fungi Phytophthora cinnamomi and P. cactorum.
Phytopthrhora cactorum, which is widespread in temperate regions is known
to infect at least 154 genera of vascular plants in 54 families. In the Midwest
it has been reported attacking Catawba rhododendron (R. Catawbiense), rosebay
rhododendron (R. Maximum) and azaleas (R. spp.). P. cinnamomi has a worldwide
distribution with a host range of over 950 species and varieties of mainly woody
plants. In the Midwest it is known to infect California rosebay (R. Macrophyllum)
and Hiryu azalea (R. obtusum). Phytophthora parasitica is a common root-rotting
fungus of greenhouse azaleas. In addition, several species of Pythium, which
are common soilborne fungi, may also be involved in the root-rot syndrome, but
their relative importance is presently unknown. Phytophthora root rot is most
widespread and severe in nurseries on 1- and 2-year old plants. It is most common
on field-grown plants growing in heavy, poorly drained soils. Disease development
is favored by high soil moisture and soil temperatures of 80 F (26.7 C) and
above. The disease is most serious during June, July and August. Temperatures
of field soils and container media during the summer range from as low as 59
F (15 C), the minimum temperature needed for infection, to as high as 122 to
131 F (50 to 55 C) at the sun-exposed walls of containers.

Figure
2. Rhododendron root rot caused by Phytophthora cactorum (courtesy
R.K. Jones).
|
Phytophthora dieback (Figures 4, 5, and 6) is a distinct phase of the
Phytophthora disease syndrome. The phase on rhododendrons is caused by
several species of Phytopthora including P. cactorum, P. citricola and
P. parasitica. In addition, P. cinnamomi, P. citrophthora, P. heveae and
P. lateralis have occasionally been isolated from rhododendron tissue
with dieback symptoms. Dieback of azaleas is caused primarily by P. parasitica.
Losses from both root rot and dieback in the Midwest have risen with
the increased use of containers for production. Temperatures are higher
in containers than in the field and overwatering is more likely to occur.
Losses are highest in containers with poor drainage where there is an
excess of soil, sawdust or other fines in the mix. They are lowest when
plants are grown in bark mixes with an air volume of 20 to 25 percent.
|
Back to Top
Symptoms
|
Root rot. At early stages of root rot in rhododendrons, some dull
yellowing and dwarfing of the leaves and retarded shoot growth may occur.
Affected leaves commonly roll downward and inward and eventually wilt
(Figures 1 and 2). The tips of shoots may die back (Figure 3). Such early
symptoms can easily be confused with a nutrient deficiency or drought
stress. At first, single branches may wilt during the heat of the day
and recover at night.
Infected roots are reddish brown and brittle. Severe damage to the root
system greatly reduces the uptake of water and nutrients resulting in
low plant vigor and general plant wilting, dieback and death. When conditions
favor disease development, young root rot-susceptible azaleas and rhododendrons
may wilt and die within a week to 10 days during the heat of summer. Wilt
and dieback symptoms may be rapid if extensive root colonization is followed
by hot dry weather. However, symptoms may take several months or years
to develop in a cool damp climate and infected but symptomless plants
are an important worldwide means of spreading the pathogens. Species and
cultivars of rhododendrons and azaleas differ in their susceptibility
to P. cinnamomi and infected but symptomless (tolerant) plants may be
a significant source of disease spread.
|

Figure
3. Greenhouse azalea infected with Phytophthora cinnamomi root
rot.

Figure
4. Bark has been removed from azalea plants to expose the wood discolored
by Phytophthora cinnamomi (courtesy D.F. Schoeneweiss).
|
|

Figure
5. Rhododendron roseum elegans infected with dieback caused
by Phytophthora citricola (courtesy R.C. Lambe).

Figure
6. Dieback of azalea caused by Phytophthora cactorum (courtesy
R.K. Jones).
|
Root rot may be diagnosed in plants with advanced symptom development
by scraping a section of bark from the main stem of the plant near the
soil line. A reddish brown to dark brown discoloration of the wood is
a symptom of Phytophthora. Discoloration of the wood may extend well up
into the main stems (Figure 4).
Dieback. Circular-like, water-soaked areas that turn chocolate
brown within a day or two form on young leaves of rhododendrons and azaleas.
Within 3 to 5 days the expanding lesions dry out, become brittle, with
infected leaves curling inward and downward but generally remaining attached
to the stem.
Infection often grows into the petioles and into stems where it produces
a brown, wedge-shaped canker (Figure 5). Entire shoot tips wilt (Figure
6), die back (Figure 7) and turn brown within 5 to 7 days. Young plants
with only 4 to 6 inches of new shoot growth may die within a week.
Initial infections develop on expanding rhododendron buds; young leaves
and young stems with mature leaves being resistant. The Phytophthora fungus
may grow from infected stem tissue through petioles into older mature
leaves along the midrib and then spread into adjoining tissue to form
an extended, V-shaped brown area. Infected mature leaves usually drop
off within two weeks. As the stem tissue of rhododendrons matures, disease
development slows.
In azaleas both young and mature stems are colonized by dieback fungi,
which results in death of entire stems. Young azalea stems commonly develop
from axillary buds at the crown. Crown infections are thus much more common
on azaleas than on rhododendrons.
|
Back to Top
Disease Cycle
|
P. cinnamomi (Figure 8) survives largely as chlamydospores and
mycelium in infected root and crown tissue and infested host debris in
the soil. The fungus does not generally overwinter in Illinois in the
absence of host plants. During periods of high soil moisture, sporangia
are produced (Figure8a and b), the contents of which divide (Figure 8c)
to form 10 to 30 motile zoospores (Figure 8d). The zoospores swim in water
films between soil particles and are attracted by root exudates to nearby
host roots where they encyst, germinate, and invade the roots both inter-
and intra-cellularly. Zoospores attack roots just behind the root cap.
Infection of rhododendron and azalea roots probably occurs when soil temperatures
are 59 F (15 C) to 83 F (28 C) with the optimum about 72 F (22 C). Soil
water is the critical factor in infection. Young actively growing plants
with a high proportion of feeder roots are especially susceptible to P.
cinnamomi as are roots damaged by high temperatures and severe water or
salt stress.
Sporangia are produced in large numbers when the soil is just below saturation
and the soil temperature is 59 F (15 C) to 95 F (35 C) with an optimum
at 75 F (24 C) at a pH of 5.5. P. cinnamomi produces large, non-papillate,
non-deciduous sporangia which usually proliferate internally (Figure 8b).
The usual temperature range for mycelial growth is between 38 and 93 F
(3 to 34 C) with an optimum of 75 to 83 F (24 to 28 C). P. cinnamomi
is a heterothallic species which forms sexual spores (oospores). This
may require two mating types (A1 and A2) or an interspecific cross with
the opposite mating type of another species such as P. cryptogea. The
A2 type of mycelium can also produce oospores homothallically if provided
with a stimulator substance released by host plant roots. Oospores are
rarely produced in nature.
Sporangia and encysted zoospores can survive in moist soil for several
weeks but, in the absence of host roots, when soil water potentials and
temperatures are sub-optimal for sporangial production, chlamydospores
and possible oospores serve as survival propagules. Germination of chlamydospores
is stimulated by organic nitrogen and some root exudates, but the conditions
that influence oospore germination have not been determined.
Rapid dissemination of P. cinnamomi occurs when it is introduced
into a new crop of rooted cuttings in irrigtion water or with infested
"soil" or host debris. It has been isolated from ponds and irrigation
water into which surface water has drained from infected rhododendron
or other susceptible plantings.
|

Figure 7.
Dieback (Phytophthora cactorum) of rhododendron (PA State Univ.
Extension Service).
|
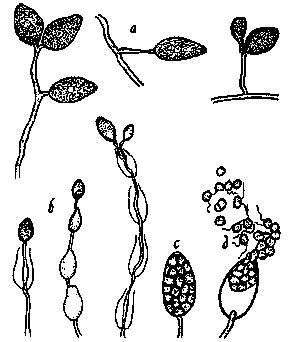
Figure 8.
Phytophthora cinnamomi as it would appear under a high-power
microscope: a, non-papillate sporangia; b, sporangia which have proliferated
internally; c, a sporangium where contents have divided up to form zoospores;
d, sporangium releasing motile zoospores (drawing by L. Gray).
|
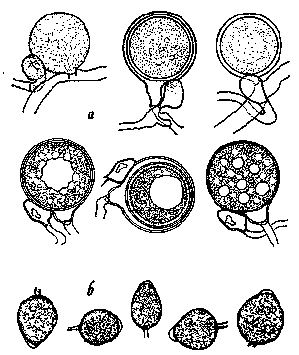
Figure 9.
Phytophthora cactorum as it would appear under a high-power microscope:
a, oospores of varying maturity with attached antheridia; b, sporangia
(drawing by L. Gray).
|
Spread of P. cinnamomi from infected to nearby healthy plants is greatest
when containers are set on black plastic and the least when they are set on
a 2- to 3-inch layer of gravel and/or coarse pine bark. However, splash dispersal
from the surface of the container medium to nearby plants also has been observed
and could play a role in the spread of the pathogen. After a plant is killed
by P. cinnamomi, viable propagules of the fungus in the soil and plant
debris gradually decline to undetectable levels over a period of 21 months.
All four types of spores produced by P. cinnamomi may be involved in
infection. Soil moisture plays a key role in the infection process. The most
rapid build-up of inoculum is restricted to freestanding water in puddles, saturated
soil, the base of containers, and flowing water on the soil surface.
A soil pH of 4.0 controls Phytophthora root rot because sporangium production
is inhibited. Such a low pH is not practical in nurseries, however, because
plant growth is too slow at this level. The components of container mixes greatly
affect the disease cycle of soilborne species of Phytophthora. Media
with peat as the only component is conducive to infection and disease development.
Media amended with tree bark release inhibitors that reduce sporangium production
and the release of zoospores. Chlamydospore production and/or survival are apparently
not affected by these inhibitors; however, bark-amended media are suppressive
to Phytophthora root rot.
P. cactorum (Figure 9) survives outside living hosts as oospores (Figure
9a) formed in diseased tissue which remain viable for several years. In free
water and a temperature of 50 to 68 F (10 to 20 C) oospores germinate to produce
sporangia (Figure 9b) which in turn produce motile zoospores. Zoospores are
probably the main infective agents although the germ tubes of sporangia may
also initiate infection. A film of water is required for infection to occur.
Periods of cloudiness after heavy rainfall or irrigation are ideal for infection.
P. cactorum and other Phytophthora dieback fungi are disseminated
by mass flow of soil water, irrigation, or by rainsplash, and thus are associated
with wet conditions or with poorly drained soils. Invasion is primarily through
wounds, unprotected surfaces, and natural openings such as stomata and lenticels.
Sporangia may develop in tremendous numbers from diseased tissues and give rise
to further cycles of infection.
The disease cycle of Phytophthora dieback is presumed to be the same
in azaleas and rhododendrons. It is thought that irrigation or rainfall splashes
zoospores and sporangia onto the current season's foliage from the base on which
container plants are placed or from the surface of the container medium. In
nurseries, the average height of lesions on plants is about a foot above the
container base, the range is 6.8 to 24 inches above the base. Fallen, air-dried
infected leaves do not serve as a source of inoculum for secondary dissemination
or survival of P. cactorum or other dieback fungi.
Back to Top
Control
A comprehensive disease management program is essential to control Phytophthora
root rot and dieback. Control measures are divided into those for (a) landscape
plantings and (b) propagators and nurserymen. Control includes a
series of proper cultural practices combined with disease resistance and the
use of fungicides. Control is complicated by the very wide host ranges of the
pathogens, the variable and sometimes lengthy period between infection by root
rot fungi and the appearance of foliar symptoms, and the longevity of chlamydospores,
oospores and mycelium in soil and root debris, often at a considerable depth.
Disease control depends on the routine manipulation of many control measures
to prevent infection and restrict the spread of disease.
A. Landscape Plantings
- Purchase only disease-free, balled and burlapped (B&B) or container-grown
rhododendrons and azaleas from a reputable nursery where strict sanitation-indexing
production procedures (see below) for macropropagation have been followed.
Be sure the plants have white, healthy rootlets and good foliage color free
of leaf lesions.
- Plant in sites amply protected from wind and direct afternoon sun. Avoid
southern exposure. These plants do best on the east or north sides around
buildings. In open areas they prefer filtered shade or alternating sun and
shade as found under tall, deep-rooted trees such as oaks, hickories, pines,
and hemlocks. Locate plants where the site does not dry out too quickly and
is well-drained, where there is adequate air circulation, and away from competing,
shallow-rooted trees such as maples, elms, poplars, and birches.
Avoid planting in unusually narrow areas between a house foundation and
a paved walk; frost pockets, such as a closed valley or depressions in which
cold air settles; areas where water may stand for several days injuring
or killing the roots; locations where they will be exposed to strong winter
sunshine and subject to sudden temperature fluctuations such as sun to shade
in late afternoon.
Space the plants far enough apart from other plants or building so they
will not be crowded when mature. A good plan is to place small azaleas two
feet apart. After 3 or 4 years, when they start to crowd each other, remove
alternate plants and replant in another location, thus giving the remaining
plants room to develop.
- Rhododendrons and azaleas require special soils for their best growth.
Light, porous, well-drained soils with an abundance of organic matter is a
must for satisfactory growth. In addition, the soil must be acid (pH 4.5 to
5.5). Poor growth, yellowish foliage–often due to iron chlorosis–and
dying of branches will occur if the soil is not sufficiently acid. For poorly
aerated, heavy clay soils use a mixture of 50 percent leaf mold or peat moss,
25 percent coarse sand, and 25 percent soil. In such soils drainage should
be supplied by drain tiles or by placing a layer of crushed stones or cinders
under at least two feet of well-prepared soil mix in which the plants are
set. Another solution is to construct raised beds that will provide good drainage.
If the natural soil is alkaline, this can be corrected by adding acidifying
materials. Check with your University of Illinois Extension horticulturist
or local Extension office on what to use and how it should be applied. Iron
or ammonium sulfate are good choices. For more slowly acidifying agents,
spent tan-bark, well-rotted hardwood sawdust, and rotted oak leaves can
be used. These materials also increase the organic matter in the soil which
tends to maintain a constant supply of moisture for roots.
- Apply a 3- to 6-inch mulch over the soil surface after planting. Use tree
bark, weathered wood chips or shavings, acid leaf mold, or weathered sawdust.
Then cover the surface with oak leaves or pine needles when available. Oak
leaves or pine needles may be used alone to a 3- to 6-inch depth. Add mulch
when growth has hardened in the fall, after deciduous plants have dropped
most of their leaves.
Mulching will insulate the soil from sudden temperature freezes and reduce
the depth to which freezing occurs. Leave mulches in place during the growing
season for insulation, moisture-retention, and to maintain more favorable
conditions in the root zone. Mulches tend to decompose rapidly in fertile
soils, therefore add new mulch each autumn. Use 10 to 12 inches of dry leaf
mulch (oak is preferable) or enough pine needles to maintain at least a
3-inch layer.
- Rhododendrons and azaleas are shallow rooted and ample moisture must be
maintained about their roots at all times. In late November water the ground
thoroughly to prevent its drying out during winter.
- Fertilize annually according to recommendations of Extension horticulturists
or your nearest Extension office. Nitrogen in the ammonium form, such as ammonium
sulfate, is preferred rather than the nitrate form. Young rhododendron and
azalea leaves with 1.8 to 2.5 percent nitrogen are highly susceptible to infection
by dieback fungi. Those with 0.7 percent nitrogen have fewer and smaller lesions
that do not expand.
- Cultivars in 10 hybrid azalea groups vary from highly resistant to highly
susceptible to P. cinnamomi. The most resistant cultivars in a large test
were Formosa, Fakir and Corrine Murrah. The Indica hybrids as a group are
the most resistant and Carla hybrids the most susceptible. The azalea species
Rhododendron poukanense has good resistance and cold hardiness and
is commercially acceptable.
The following azalea cultivars are reported as having resistance:
| Curlin hybrid: Fred Cochran; |
| Glenn Dale hybrids: Fakir, Glacier, Merlin, Polar Seas; |
| Gable hybrid: Rose Greeley; |
| Kurume hybrid: Morning Glow; and |
| Satsuka cultivars: Higasa, Shin-ku-gen, Pink Gumpo, and Eiken. |
The resistance of azaleas and rhododendrons can be greatly reduced by moisture
stress caused by too much or too little water. Most rhododendron hybrids and
the native rhododendron species, R. maximum, are susceptible to Phytophthora
dieback. In a survey of nurseries, this disease was greatest in the cultivars
Chionoides White, Catawbiense Album and Nova Zembla and lowest in Roseum Elegans,
Scintillation, and PJM.
- Where Phytophthora root rot and dieback have been a problem in the past,
or are expected to cause injury, apply a preventive fungicide as a
spray, or drench around plants to saturate the soil. Repeat at 2- to 12-week
intervals in spring and autumn; or blend granules into the soil mix just before
planting. There are no therapeutic fungicides. Infected nursery stock
may eventually become diseased in the landscape even if it is repeatedly treated.
When using a fungicide, carefully follow label directions. Recommended fungicides
to control Phytophthora root rot and dieback are given in Illinois Homeowners'
Guide to Pest Management. This circular is revised annually.
Back to Top
B. Propagators and Nurserymen
- Nursery stock should be grown under high standards of santation using a
clean (chlorinated) water supply. Propagate only from cuttings taken from
disease-free mother plants. Take cuttings high on stock plants where soil
has not splashed. This avoids introducing root rot and dieback pathogens to
the rooting bed. Soil particles should not adhere to the leaves or stems of
cuttings. Domestic or foreign imported stock should be segregated in the nursery
and preferably grown in containers for several months to be sure the plants
are healthy and free of infection. Potting compost and propagation benches,
beds, containers, and tools should be steam-pasteurized (air-steam mixtures
at 160 F for an hour). Field beds may be fumigated with methyl bromide, methyl
bromide-chloropicrin mixtures, Vorlex or Vapam prior to planting. These
chemicals are limited to the depth of fumigant penetration. They are highly
toxic materials, many of which are restricted, and should be carefully applied,
strictly following the manufacturer's directions. Soils usually become reinvaded
within several months of fumigation and then root rot and dieback progress
as they did before treatment. In nurseries, infested soil is often fumigated
with methyl bromide or a methyl bromide-chloropicrin mixture and thereafter
treated with organic amendments to prevent or reduce recolonization of the
soil by species of Phytophthora. This practice induces suppressiveness
of the soil.
In the propagation house, direct contact of potting medium with the ground
should be avoided by using screen-bottom benches. Prior to filling benches
and sticking cuttings, all wood surfaces should be scrubbed down with a disinfectant
such as fresh liquid household bleach (1 part bleach in 5 parts of clean tap
water). Containers used in potting operations should be new or fumigated prior
to reuse and never stored directly on the ground.
- A well-drained potting mix is essential. A major component of nursery potting
media is composted hardwood bark or pine bark. Certain inhibitory chemicals
that suppress root rot fungi are formed during the composting of hardwood
bark. Apparently pine bark is suppressive to root rot fungi because of the
excellent drainage. In general, avoid soil since it retains water too long,
may contain spores of Phytophthora, and makes containers too heavy to handle
conveniently. When soil is used as a component of the potting mix, it should
be steamed or fumigated prior to use. Peat moss or weathered sawdust alone
should also be avoided. Media prepared with very fine bark particles can also
be conducive to disease. The potting medium should contain 10 to 15 percent
air-filled pore space. To assure adequate drainage the water percolation rate
of potting media must remain at an inch per minute throughout the production
cycle.
- It is important to place container-grown plants on a well-drained base such
as 3 to 4 inches of gravel, a similar neutral aggregate or pine bark to prevent
splash dispersal of zoospores and sporangia. Regulate the irrigation system
to avoid the formation of puddles or periodic overwatering.
Container areas should be graded prior to use to provide surface water drainage
away from the growing area. Locate water control ditches throughout the nursery
so run-off water during irrigation or heavy rainstorms will be channeled away
from container areas, potting media, storage piles, and other potting supply
areas. Installing concrete pads for storing container media and pots, mixing
and potting (canning) operations is a good investment. This protects against
introducing root rot and dieback fungi accidentally into the nursery operation.
Host debris, such as prunings and fallen infected leaves, should be removed
from the production area between crops to reduce the survival of Phytophthora.
Group plants in the nursery by container size and water requirements to avoid
the danger of overwatering. Commercially-available irriometers can be used
to monitor the soil-water status so water is applied only when needed.
- Field plants should be grown in well-drained soil. Routinely inspect plantings
and either destroy infected plants (with root rot) or skillfully prune and
burn shoots with dieback. Prune diseased tips well below the cankered and
shriveled part. Prevent the movement of Phytophthora-infested soil on footwear,
tools and equipment. In infested field soil the incidence of P. cinnamomi
can be reduced, if not eliminated, by cropping with a nonsusceptible host,
leaving the land fallow for at least four years or incorporating organic nitrogen
into the soil.
- Fertilize to avoid excessive concentrations of nitrogen in the foliage.
- If dieback has been a problem, lessen shade if possible and increase air
movement.
- When possible, grow resistant species and cultivars of azaleas as given
under A7, Landscape Plantings.
- Preventive fungicides active against species of Phytophthora and
Pythium can be applied to healthy, container-grown plants as sprays,
drenches to the soil surface, or by incorporating granules into the potting
medium at canning.
In all methods of application the fungicide(s) is/are effective only when:
(1) it is applied prior to infection, and (2) concentration of the
fungicide remains at or above the effective rate. This means that repeated
applications will be needed, usually at 2- to 8-week intervals, throughout
the growing season. Emphasis, however, should not be placed on fungicides
but on correct cultural practices. The use of fungicides in the nursery is
important to prevent the spread of Phytophthora from infected to healthy
plants. If root rot or dieback symptoms should develop on a few plants in
a container block, applications of fungicide to all plants in the block is
recommended to prevent further spread to healthy plants. Applied as eradicants,
once plants are infected, fungicides will arrest but generally not
kill the fungi or fungi in infected roots and soil. Suggested fungicides to
control Phytophthora root rot or wilt and dieback are given in Illinois
Homeowners' Guide to Pest Management, which is revised annually.
Back to Top
For further information concerning
diseases of crucifers and other vegetables, contact Mohammad Babadoost, Extension
Specialist in Fruit and Vegetable Diseases, Department of Crop Sciences, University
of Illinois at Urbana-Champaign. University
of Illinois Extension provides equal opportunities in programs and employment.
|



