Reports on Plant Diseases |
| RPD No. 911
- Root Rots of Pea
|
April 2002
|
[ Symptoms
] [ Disease Cycle ] [ Control
]
Root rots occur wherever peas are grown
in the world. Crop losses are probably greater from root diseases than
from any other type of pea disease. Damage is usually most severe in wet
seasons. Low-lying areas and slowly drained fields or gardens suffer greater
losses than well-drained, fertile ones. Root rot generally occurs in patches
that progressively enlarge during the season. Root rot may start when
the pea plant is in the pre- or postemergent seedling stage. Death soon
follows such early infections, resulting in a poor stand. Root decay generally
begins on the fine feeder roots and progresses gradually to the main taproot.
Sometimes, however, the taproot is the first to be attacked. In some cases
all roots are destroyed, leaving only remnants below the attachment of
the seed. Root-rotting fungi can also reduce the quality of shelled peas.
Infected plants produce peas which are irregular in size and variable
in maturity with a lowered sugar content. Root rot of pea may be caused
by any one or a combination of several common soil fungi. The most common
pathogens are Aphanomyces euteiches, Pythium ultimum, Fusarium
solani f. sp. pisi, and Rhizoctonia
solani. Other fungi that can be associated with pea root
rots include Thielaviopsis basicola, Fusarium oxysporum, Ascochyta
inodella, and Sclerotinia sclerotiorum. Aphanomyces
euteiches and Pythium ultimum
are probably the most important of the fungal species reported. It is
often difficult or impossible to differentiate the common types of root
rot, especially in an advanced stage or late in the season. At this time,
it may be difficult to determine whether root rot or a wilt disease has
killed the plants. Pea plants damaged by root rot usually may be pulled
from the ground easier than those affected by wilt, whose roots are still
intact. The common root-rotting fungi may attack the same plant at the
same time. Species of Pythium, as well as lesion,
stunt, and other nematodes are all commonly a part of the disease complex
and can increase the severity of root rot. If plants in the field or garden
are stunted, or the leaves are pale yellow, dig up some of the plants,
carefully wash off the soil, and examine the roots for decay.
Back to Top
SYMPTOMS
The symptoms for the four common types of root rot are as follows:
-
Aphanomyces root rot
can infect plants at any age. The central part of the taproot (vascular
tissue) separates easily as a long, fiber-like string from the outer,
softened, water-soaked portion (cortex) of the root when the plant
is pulled from the soil. The fine branch or feeding rootlets are killed.
The decay may extend up the stem to slightly above the soil line.
The decayed surface is often slimy in wet soils turning gray, then
yellowish or pink, and finally brownish black as secondary microorganisms
invade the diseased tissue. The leaves shrivel progressively upward
on the stem. Plants are usually stunted, and the leaves progressively
turn yellow starting at the bottom of the shoot. Pods may be few with
a reduced number of small seeds. In severe cases, the plants collapse
and die before forming any pods (Figure 1). Late-infected plants appear
almost normal aboveground and often produce normal peas. Aphanomyces
root rot is the most common and destructive disease of peas in the
Midwest.
-
Pythium commonly causes
seed rot as well as pre- and post-emergence damping-off of pea. Root
rot of older plants also occurs, and often results in root-pruning
that significantly reduces root length. Damage is most common in wet
soils and is characterized by soft rot. Roots infected with Pythium
are typically light brown in color and soft and watery to the touch.
Infected plants are frequently stunted and pale green to yellow in
color. Although it primarily causes a seed rot, damping-off, and seedling
root rot, Pythium ultimum can cause a watery,
soft decay of older plants in wet soils at an optimum temperature
of 64° to 75°F (17° to 23°C).
-
Fusarium root rot affects
mainly the taproot with infection starting close to where the seed
is attached. Reddish brown streaks form in the primary and secondary
roots and later merge. The external portion of the stem shows brick
red, dark reddish brown, or chocolate-colored lesions. The advancing
lesion may be wedge-shaped with the point upward. The central part
of the taproot is a deep red. Plant growth is stunted, the foliage
turns grayish, then yellow, the lower leaves wither, and the plant
eventually dies. The lower stem is often girdled, causing the plant
to fall over. Pythium ultimum is often found
in Fusarium-infected roots and vice versa.
-
Rhizoctonia root rot
can attack plants at any stage of growth. Seeds may turn dark brown
and decay. Water-soaked, then reddish brown to brown lesions form
in the seedling epicotyl and hypocotyl. The growing point may die
as it emerges from the soil. Seedlings damp-off or recover to produce
a normal plant. On older plants, scurfy, reddish brown, sunken lesions
form on the underground stem and roots. The stem may be girdled causing
severe plant stunting and yellowing. The brown, thread-like filaments
(mycelium) of the causal fungus may be seen with a hand lens on the
surface of the lesion or canker.
Back to Top |

Figure 1.
Healthy pea plant on left and plants infected with Aphanomyces root rot
on right.
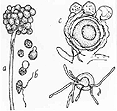
Figure
2. Aphanomyces euteches as seen under a highpower microscope:
(a) primary spores encysted at the tip of a sporangium; (b) evacuation
of encysted zoospores and motile stage after evacuation; (c) oospore with
antheridia after fertilization; (d) oospores germination with formation
of germ tubes (drawing by L. Gray).
|
|
All root-rotting fungi are “soil inhabitants”
and once introduced will persist in the soil for several years or longer.
Rhizoctonia root rot will damage peas at relatively
low soil temperatures (65°F or 18°C) but is most aggressive under
warmer conditions (76° to 86°F or 24° to 30°C). Rhizoctonia
infection and disease development can occur over a wide range of soil
moistures. Fusarium root rot, on the other hand, is favored by higher
soil temperatures (optimum between 77° and 87°F or 25° to
30°C) and moderate soil moisture. Aphanomyces root rot, most damaging
at soil temperatures between 72° and 82°F (22° to 27°C),
is favored by excessive soil moisture. It is most serious when a cool,
wet spring is followed by an early, warm, dry summer. Although P.ultimum
primarily causes a seed rot, damping-off, and seedling root rot, it can
cause a watery, soft decay of older plants in wet soils at an optimum
temperature of 64° to 75°F (17° to 23°C).
The Aphanomyces fungus (Figure
2) also attacks alfalfa, snap beans, cowpea, spring vetch, sweet clover,
spinach, sweet pea, and some weed species. The fungus produces large numbers
of asexual, microscopic spores (zoospores) capable of swimming in soil
water before contacting a susceptible pea root. If contact is made, the
zoospores attaches to the root and produces a germ tube that penetrates
the root. The developing mycelium invades and ramifies through the host
tissues. Tremendous numbers of thick-walled sexual spores (oospores) are
formed in root tissue infected by Aphanomyces. As the roots decay the
oospores are released into the soil. The oospores are highly resistant
to adverse environmental conditions and remain viable in soil organic
debris for up to 10 years or longer in the absence of a pea crop. When
they germinate, the oospores form hyphae or sporangia. The sporangia,
which closely resemble the tubelike hyphae, in turn produce the primary
spores which cluster (encyst) at the mouth of the sporangium before releasing
their zoospores (Figure 2).
The spores of Aphanomyces are readily carried over long
distances by surface-drainage and splashing water, in movement of soil
from one area to another, and in infected seed. The oospores can be mixed
with the seed and be introduced into new fields or gardens when such contaminated
seed is planted. The zoospores are produced and germinate in abundance
between 57° to 68°F (13° to 20°C) with some germination
occurring as low as 48°F (8°C) and as high as 86°F (30°C).
The optimum temperature for infection occurs at 61°F (16°C) but
symptom development is markedly more rapid at warmer temperatures.
Fusarium solani f. sp. pisi
produces three types of microscopic spores: small, one-celled, elliptical,
microconidia; much larger, septate, slightly curved macroconidia; and
thick-walled, rounded chlamydospores (Figure 3). The chlamydospores are
resistant to unfavorable environmental conditions and can persist in soil
for 5 years or more in the absence of peas. Any activity that moves infested
soil from one area to another spreads the fungus. Fusarium root rot is
most severe at soil temperatures of 79° to 82°F (26° to 27°C)
and at moderate soil moistures whereas Pythium ultimum
is more of a problem in cool (64° to 75°F), wet soils. Peas planted
in soil infected with both Fusarium and
Pythium ultimum are more severely attacked than plants infected
with only one pathogen at any soil temperature or moisture level. Germination
of overwintering chlamydospores is stimulated by the release of nutrients
from germinating pea seeds. Maximum germination of chlamydospores occurs
20 hours after seeds are planted in soils with a minimum of 9 percent
soil moisture. Hyphae from germinated chlamydospores penetrate the epicotyl
and hypocotyl through natural openings (stomates), wounds, or directly
through the epidermis. The fungus then invades the root system where it
causes a reddish brown discoloration of the vascular system. As tissues
senesce, spores are formed abundantly in and on pea debris.
The Rhizoctonia fungus (sexual
stage Thanetophorus cucumeris) is common in
most soils and attacks hundreds of different plants. It is subdivided
into many strains and anastomosis groups that differ in the hosts and
tissues they attack. Rhizoctonia persists indefinitely in soil as a saprophyte
and survives extremes in temperature and soil moisture as small, brown,
rounded sclerotia. Under favorable conditions the sclerotia germinate
by producing delicate hyphae (Figure 4) that grow through the soil and
invade roots directly, through wounds or natural openings when sufficient
soil moisture is present. The Thanetophorus
sexual state plays little or no part in the disease cycle.
|
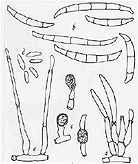
Figure
3. Fusarium solani f. sp. pisi as seen under a high-power microscope:
(a) macroconidia at the tips of spore-bearing conidiophores; (b) macroconidia;(c)
chlamydospores; (d) mono con idia; (e) two conidiophores bearing microconidia
at their tips
(drawing by L. Gray).
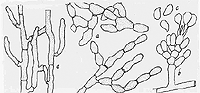
Figure
4. Rhizoctonia (Thanatephorus cucumeris) solani as seen under
a high-power microscope: (a) three types of Rhizoctonia hyphae; (b) sexual
Thanatephorus state with basidium bearing three basidiospores;
(c) basidiospores (drawing by L. Gray).
|
Back to Top
-
There is no completely satisfactory control for
the root-rot diseases of pea once the causal fungus or fungi is/are
introduced and becomes prevalent in the soil. Control measures for
all root rots are the same.
-
Plant early in fertile, well-prepared, well-drained
soil using seed grown in semiarid areas of the Pacific Northwest.
Treatment of the seed with a seed-protectant fungicide may provide
young roots some protection. For information on procedures and fungicides
recommended for seed treatment, read C1373, Midwest Vegetable Production
Guide for Commercial Growers, available from ITCS, University of Illinois
P345, 1917 S. Wright St., Champaign, IL 61820.
-
Follow a crop rotation scheme in which peas are
grown in the same area only once in 5 years or more. If A. euteiches
is the primary problem, do not include alfalfa, beans, sweet clover,
cowpeas, spinach, or vetch in the rotation. Crucifers are suitable
for rotation.
-
Field indexing, a method of determining the inoculum
potential of Aphanomyces euteiches prior to planting, was developed
at the University of Wisconsin and is useful for commercial growers.
The soil-assay or “root rot potential” procedure estimates
the amount of infective inoculum in soil and the level of disease
that can be expected if peas are planted in the field. Growers should
avoid planting in moderately to highly infested fields with an index
of 75 or higher. Information on field indexing can be obtained by
contacting an Extension Plant Pathologist at the University of Illinois,
N-533 Turner Hall, 1102 S. Goodwin Ave., Urbana, IL 61810.
-
Do not spread unfermented pea straw and other
crop refuse on fields to be planted in peas.
-
Maintain an adequate to high, balanced soil fertility
level, based on a soil test. The addition of at least 3 tons per acre
of rock phosphate, hydrated lime, or calcitic limestone may reduce
losses from root rot. The optimum soil pH is 6.5 to 7.0.
-
Avoid overcrowding, deep planting, overfertilizing,
soil compaction, and mechanical damage to the roots and stems.
-
Any cultural practice that enables peas to maintain
a good, steady rate of growth from the start helps to prevent losses
from root rots.
-
No acceptable commercial cultivars of peas are
currently available that are highly resistant to any root-rotting
fungi although some have low/moderate resistance. Planting cultivars
that perform well in Illinois may reduce the amount of stress-related
loss. Refer to publication mentioned above for more information.
Back to Top |
Information concerning insecticides, weed control, varieties, and other recommendations
can be found in the Illinois Homeowners' Guide to Pest Management, available
at your nearest Extension office.
|



