Reports on Plant Diseases |
| RPD No. 921 -
Bacterial Diseases of Beans |
June
2000 |
COMMON BACTERIAL
BLIGHT [ Symptoms ]
HALO BLIGHT [ Symptoms
]
BACTERIAL BROWN SPOT
[ Symptoms ]
[
Disease Cycle ] [ Control ]
|
There are three major bacterial diseases of common beans: common bacterial
blight (and its variant, fuscous blight), halo blight, and bacterial brown
spot. These diseases have had major impact on bean production.
In rainy and windy seasons it is not unusual to suffer a loss in yield
of 10 to 20 percent or more from these diseases. However, the greatest
loss is in a reduction in quality. When pods are infected only one percent
"serious blemishes" are allowed for grade A cut beans. Processing
beans are graded substandard when four percent is exceeded. Seriously
diseased commercial crops are often not harvested.
In many situations, symptoms of the different bacterial blights appear
very similar. For example, halo, common, and fuscous blights are difficult
to distinguish at temperatures above 75 F (24 C). Laboratory isolation
is nearly always necessary for positive identification of the bacterial
species.
COMMON BACTERIAL BLIGHT
Common bacterial blight, caused by the bacterium Xanthomonas campestris
pv. phaseoli, affects green or snap, wax, field, and lima beans as well
as the scarlet runner, mung, Tepary, urd, moth, hyacinth, and civet or
Sieva beans, the Washington or white-flowered lupine, and fenugreek (Trigonella).
Fuscous blight, caused by fuscous variant of the bacterium, occurs on
field beans, civet, and scarlet runner beans. Common blight affects the
foliage and pods of beans and causes significant losses in both yield
and seed quality. The disease typically develops when contaminated seed
is planted, when plantings are made in fields with a history of the disease,
and when the climate is consistently hot and wet or humid.
Symptoms
Common and fuscous blights are commonly first seen as small, angular,
light green, water-soaked or translucent spots (lesions) on the leaves.
During warm, wet conditions the lesions rapidly enlarge and merge. As
they develop the centers become dry, brown, and surrounded by a distinct,
narrow, zone of yellow tissue. In highly susceptible varieties, the lesions
continue to expand until the leaves appear scorched or sun scalded (Figure
1). Such leaves soon become ragged and torn by wind and rain. Later, they
wither and drop off.
Pod lesions start as round, water-soaked dots that enlarge, merge, dry,
and form sunken, irregular, frequently reddish brown blotches (Figure
2). When severe, entire pods may be badly shriveled and die. Seeds in
such pods either fail to develop or are shriveled. Seeds in less severely
affected pods develop normally and show no signs of disease. Other seeds
may become slightly wrinkled. When seeds containing the bacteria are planted,
many fail to germinate. Those that do germinate produce seedlings with
blight lesions on the cotyledons, stems, and first true leaves.
The stems of seedlings may have water-soaked, sunken areas that enlarge
and develop into reddish streaks. Any time during the season affected
stems commonly crack and become girdled by water-soaked cankers or rot.
The tops may break over during a rain or strong wind. In humid weather,
a yellowish bacterial ooze, that later dries to form a crust, may be evident
on the lesions on infected pods, leaves, stems, and cotyledons.
|

Figure 1.
Common bacterial blight of bean
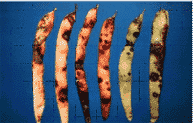
Figure 2.
Common bacterial blight on pods.
|
|

Figure 3.
Halo blight of beans.
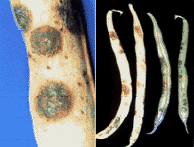
Figure 4.
Left, Lesions and bacterial crust of halo blight on pod (courtesy A.W.
Saettler); Right, Halo blight on pods (courtesy A.F. Sherf).
|
HALO BLIGHT
Halo blight, caused by the bacterium Pseudomonas syringae pv. phaseolicola,
affects green or snap, wax, lima, scarlet runner, and civet beans, and
kudzu.
Symptoms
Leaf symptoms appear several days after infection as small (1/8 to
1/4 inch, or 3mm), water soaked, tan brown, angular spots, each surrounded
by a relatively wide, diffuse, pale green to yellow green tissue (halo).
Halos do not develop or tend to disappear if the temperature is above
70 to 75 F (21 to 23 C). The spots then become reddish brown to brown,
and dry. Infection foci generally remain small. In cases of severe leaf
infection, plants develop a generalized systemic chlorosis. The systemic
chlorosis is particularly pronounced at 64 to 73 F (18 to 23 C). Leaves
with systemic infections lack halos. The intervinal tissues of these
leaves are yellow, with the veins remaining dark green. At 7-10 days
after infection, bacteria ooze from the sub stomatal cavities to give
lesions a greasy, water-soaked appearance. Bacteria are thus available
for secondary spread of the disease. Infected plants may defoliate,
wilt, and die. Diseased plants may be dwarfed, with the upper leaves
crinkled and mottled. Dark green, water-soaked areas may also be visible
on the stems.
Young seedlings infected with halo blight develop a chlorosis (yellowing),
which may be general or confined to the young trifoliate leaves at the
top of the plant. The chlorosis is caused by a toxin produced by the
halo blight bacterium.
Pod infections develop rapidly under cool, wet conditions resulting
in oval to circular, dark green, water-soaked, "greasy" lesions
to 3/8 inch (9 mm) in diameter. Halos do not develop around pod lesions.
As pods mature and turn yellow, pod lesions may remain green and may
exhibit crusty bacterial ooze on the surface. Developing seed may be
shriveled or discolored if lesions expand to involve the pod surface.
With age the lesions become slightly sunken and reddish brown.
BACTERIAL BROWN SPOT
Bacterial brown spot is caused by the bacterium Pseudomonas syringae
pv. syringae (Figures 4 and 5). In addition to attacking lima, green
or snap, and wax beans, the bacterium infects a wide range of host plants.
These hosts include civet, hyacinth, yard-long, and broad beans, soybeans,
cowpea, clovers, alfalfa, corn, sorghums, Sudangrass, buckwheat, kudzu,
lilac, ash, poplar, Forsythia, wild and cultivate stone fruits (Prunus
spp.), apple, pear, Citrus species, rose, flowering stock, hibiscus,
jasmine, Florida velvetbean, and many others. Fortunately only bean
isolates are highly virulent on beans.
Symptoms
On lima beans, small, light gray spots with yellowish brown borders
develop on the upper leaf surface. Water-soaking is not as pronounced
as it is in common and halo blights. Later, the gray centers may crack
open or drop out, leaving small holes. The veins on the under leaf surface
turn red or reddish brown. The spots on the stems and pods are somewhat
more elongated than those on the leaves. In severe cases much of the
foliage may be killed.
On green and wax bean leaves, small (1/8 to 3/8 inch or 3 to 9 mm in
diameter), oval, brown lesions surrounded by a narrow, yellow-green
halo develop. The lesions may merge and their centers fall out giving
leaves a tattered appearance. When the bacterium becomes systemic, tan
sunken lesions with a reddish brown margin form on the stems and petioles.
Bacterial exudate (ooze) and water-soaking are rare prior to lesion
development. Pods develop somewhat circular, dark green, water-soaked
areas which gradually enlarge, suddenly become depressed and tan, then
dark brown with a distinctive, reddish brown margin. Pods infected when
young are bent at an acute angle or are twisted. Bacterial ooze is white
to cream colored
|
|
Disease Cycle
The disease cycles of all three pathogens are very similar. The bacteria
are all disseminated in infected seed and from plant to plant and field
to field by wind-driven and splashing rains, sprinkler irrigation, surface-drainage
water, insects, birds, large animals, humans, farm machinery, tools, and
other agencies. The bacteria may survive for 6 to 18 months in plant refuse
(on or above the soil surface and under dry conditions), in bean cull
piles within or near fields, on volunteer plants from a previous crop,
and even on the surface of weeds. Long-distance spread is generally by
seed, but there is evidence that minute aerosol particles from rainsplash
carrying the bacteria can float in the air for more than two hours. Windborne
particles carrying bacteria could move many miles during rainstorms.
There are two types of field infection. Primary infection involves the
invasion of bean seedlings as they emerge from the soil. This occurs when
the seed is infected or when an emerging seedling comes into contact with
infected plant material. Secondary infection is due to spread from a primary
source to other growing plants; often involving only one or two isolated
plants, or it can cover an entire field or area. In severely diseased
fields nearly all infection is secondary.
Bacteria enter a plant through natural leaf openings (stomata and hydathodes),
and through wounds created by hail, blowing soil particles, insects, or
cultivator injury. The brown spot organism often infects the leaves through
rust pustules; thus, rust and bacterial brown spot are often found together
in the same lesion.
When an infected seed sprouts and the seedling emerges, bacteria ooze
to the surface of the diseased cotyledons, are splashed to a neighboring
plant, enter the stomata, and infect. Stomate or hydathode entry also
occurs from bacteria that survive on surface debris and are rainsplashed
to the leaves and stems. Symptoms appear two to five days after penetration
for common, fuscous, and halo blights. Once inside the plant the bacteria
may move systemically, by way of the vascular bundles, to the leaves,
stems, pods, and into the seed. The bacteria are carried by rainsplash
to neighboring leaves and pod surfaces. They invade growing pods largely
along the suture, and enter the seed through the vascular tissue. The
bacteria remain on or just below the surface of the seed. The bacterial
pathogens have been recovered from 3- to 14-year-old seed. The halo blight
organism was found to be viable after passing through sheep fed on diseased
bean plants.
Usually only a few seeds in a seed lot serve as a source of primary inoculum.
One infected seed in 16,000 is thought to be sufficient to supply inoculum
for a severe outbreak of halo blight where essentially every plant in
one or more fields becomes infected. Seed from clean fields can be surface
contaminated by small dust particles which adhere to the surface of the
seed during threshing and milling.
Common blight and bacterial brown spot are favored by cloudy damp weather
and relatively high air temperatures (82 to 90 F or 28 to 32 C), while
halo blight thrives under damp and cooler conditions (64 to 72 F or 18
to 22 C).
|
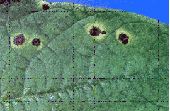
Figure 5.
Bacterial brown spot on leaf, caused by Pseudomonas syringae pv. syringae.
(Courtesy A.W. Saettler)
|

Figure 6.
Bacterial brown spot on pods, caused by Pseudomonas syringae pv. syringae.
(Courtesy H.F. Schwartz)
|
Control
1. Plant only certified, pathogen-free seed grown in semiarid areas of
Idaho and California. In these areas the humidity and rainfall is normally
too low for the bacteria to infect plants. The use of pathogen-free seed
cannot be overemphasized. All seed stock of reputable companies is treated
with streptomycin to reduce or eliminate surface contamination. The seed
is also inspected by State Department of Agriculture officials. For information
on seed treatment, read the University of Illinois Extension Service Circular
1328, Midwest Vegetable Production Guide (revised annually).
2. Do not enter fields or gardens and cultivate or handle plants wet
with dew or rain. Use a suggested herbicide to keep fields and border
areas weed-free.
3. Practice good sanitation. Whenever feasible, collect and burn (or
cover completely by thorough disking followed by deep plowing) all infected
plant debris as soon after harvest as possible. Fields subject to wind
and water erosion should be planted to a nonsusceptible cover crop before
winter. Planters, harvesters and other equipment should be sanitized by
spraying with a disinfectant such as chlorine dioxide, sodium hypochlorite,
or a quaternary ammonium compound before moving from an infected to a
blight-free field. Storage areas should also be sanitized.
4. Grow beans in the same area or field only once in three years or longer.
In the rotation exclude all beans, soybeans, and cowpeas, or other plants
affected by one or more of these diseases.
5. Foliar sprays with a fixed copper compound, applied weekly starting
four days after seedling emergence or at the first sign of disease have
given fair to good control when continued to harvest. Thorough coverage
of the plants is required. Properly applied aerial sprays are superior
to applications by ground equipment. Sprays provide good control of bacterial
brown spot and halo blight, but only moderate control of common blight.
6. Efforts to develop bean varieties resistant to bacterial blights with
acceptable quality and other characteristics have not been very successful
partly because of the appearance of new races of the organisms. Grow bean
varieties recommended for your area that are somewhat resistant to all
three bacterial diseases.
|
Back to Top
|



