Reports on Plant Diseases |
RPD No. 661 - Cytospora Canker of Poplars and
Willows
|
May 1990
|
[ Symptoms ] [ Disease Cycle
] [ Control ] [ Table 1 ]
[ Other Hosts ]
|
Cytospora canker of poplars–including aspens and cottonwoods–and
willows is caused by the fungus Cytospora chrysosperma (perfect
or teleomorph state Valsa sordida). Cytospora canker has
been associated with the decline and/or death of many thousands of valuable
ornamental trees in landscape, windbreak, and recreational areas as well
as poplar (cottonwood) cuttings in storage and nursery propagation beds.
This stem disease commonly kills Lombardy poplars (Populus nigra
cv. ‘Italica') by the time they are 10 to 15 years
old (Figure 1). The Cytospora fungus has been reported on
a number of hosts (Table 1).
The disease is usually associated with trees growing outside their normal
range or under unfavorable conditions due to a poor site, frost damage,
periods of drought, extremely cold winter weather, transplant shock, or
severe pruning (pollarding). The fungus kills areas of bark on branches
and trunks creating circular to oval or elongate sunken lesions (cankers)
(Figures 2 and 3). Frequently, as the lesions enlarge, affected stems
are girdled and the portion beyond the canker is killed (Figure 1).
|

Figure 1.
Lombardy poplar trees being killed by Cytospora canker.
|
Symptoms
|
Discrete cankers first appear on young trees as brown, slightly sunken
areas in the smooth bark of branches and trunks (Figure 3, left). These
cankers are circular to oval or irregular in shape. Frequently, as the
canker gradually enlarges, affected stems are girdled and killed. Twigs
commonly die without the formation of typical lesions. Vertical cracks
within the lesion and along the canker margins often occur in the bark
(Figures 2 and 3, right). As the cankers enlarge the diseased outer bark
may become black, brown, gray, reddish brown or yellow and sunken depending
on the host species and stage of disease development. The inner bark turns
black and sometimes gives off a foul salty odor. The sapwood appears reddish
brown to black and water-soaked. Cankers frequently start at wounds or
branch stubs or at the base of dead twigs. Cankers on large stems with
thick, rough bark may be imperceptible except for yellowish to reddish
brown spore horns (sticky, thread-like masses of spores) protruding from
bark fissures (Figure 3, right).
Highly susceptible trees, such as Lombardy poplars (Figure 1), may die
within 2 to 5 years after becoming infected. Severely infected trees usually
die branch by branch often producing sprouts at the base of the trunk
which also become infected and die.
Cytospora chrysosperma is also the primary cause of blackstem
disease of cottonwood seedlings and cuttings which causes severe losses
in nursery beds and in prolonged or improper storage. Symptoms of blackstem
occur in the fall as small lesions at the ends of cuttings or at leaf
scars and lenticels, usually on stems but occasionally on the roots. The
lesions enlarge during the winter, becoming dark brown to black and water-soaked
with distinct margins.
|

Figure 2.
Cytospora cankers on a Simon poplar in a nursery. Note the sunken girdling
cankers on the branches and trunk and the flow of gum oozing from the
dead tissues (Illinois Natural History Survey photo).
|
Back to Top
Disease Cycle
|
Cytospora chrysosperma and its perfect state Valsa sordida
is generally considered to be a saprophyte or weak parasite living on
dead bark. It can assume a parasitic role and quickly attack trees that
have been weakened by stresses such as crowding, drought, extreme heat
or cold, nutrient imbalance, transplant shock, severe pruning, fire, sunscald
injury, frost, insect or mechanical injury, herbicide damage, root-feeding
nematodes, insect damage, or infection by other pathogenic fungi. This
opportunistic fungus often inhabits apparently healthy bark and buds and
is thus in position to infect weakened tissue quickly and massively. A
canker frequently begins at a wound, branch stub, or leaf scar.
Shortly after the bark dies two types of black, pinhead-sized, spore-producing
bodies form in stromata in the outer diseased bark (Figure 2 and 3); the
pycnidia of the asexual phase (Cytospora chrysospermai)
and the perithecia of the sexual state (Valsa sordida) (Figure
4). The pycnidia are much more abundant than the perithecia. The stromata
are shaped like short cones with flattened, gray-brown to black tips that
break through the bark surface as small dark pimples or pustules (Figure
3). The pycnidia, under warm moist conditions, absorb water and swell,
exuding long, thin, coiled, thread-like tendrils of microscopic spores,
called spore horns. The yellowish to reddish brown spore horns consist
of masses of one-celled spores (conidia) in a gelatinous matrix. As these
structures dry, the conidia are released and are carried by dripping and
splashing rain, wind, insects, birds, and tree workers' hands, clothing,
and pruning tools to other trees.
The perithecia of Valsa sordida form in the same stromata with pycnidia
or in new stromata beginning in autumn and winter after the formation
of pycnidia. The perithecia are black, spherical, and several are arranged
in a ring in the lower, outer part of the stroma. Their long necks converge
to form a circle of openings in a disc which protrudes through the cracked
bark (Figure 4a and b). When the stromata are wet for a prolonged period
the asci (Figure 4c), each containing 8 ascospores, may exude from the
perithecium much like the release of conidia. The colorless, one-celled
ascospores (Figure 4d) may also be forcibly expelled into the air when
the stromata in dry bark become saturated with water.
The Cytospora (Valsa) fungus overwinters as mycelia and conidia or ascospores
in diseased bark and wood. Infection usually occurs through bark wounds
typically resulting from mechanical damage. The fungus grows through the
bark cells and the outer few rings of wood. Cankers usually develop in
the fall, winter, and early spring and enlarge slowly at low temperatures
(36 to 50 F or 2 to 10 C) and up to 40 millimeters per day at higher temperatures
(68 to 86 F or 20 to 30 C). Bark susceptibility may be induced by heating
to approximately 104 F (40 C) which is not uncommon on hot summer days.
Rapid temperature shifts in the fall and spring between warm and subfreezing
also predispose the bark to infection.
|

Figure
3. Cytospora canker of willow. Left, canker on a dwarf arctic willow
stem following transplant shock (courtesy Dr. D.F. Schoeneweiss); right,
cankers on an older weeping willow 9courtesy Dr. L.E. Dickens). Note the
fruiting bodies of the Cytospora fungus which appear as pustules in the
diseased bark.
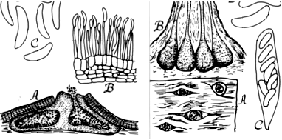
Figure
4. Cytospora chrysosperma (left) as it would be seen under a high-power
microscope. A, Section through a pycnidial stroma showing two chambers
and a pore releasing spores (conidia) from the right chamber; B, section
of the pycnidial wall showing conidiophores bearing conidia at their tips;
C, six colorless, one-celled conidia. Valsa sordida (right). A. Top view
of four perithecial stromata erupting through the bark; B, section through
a stroma showing four perithecia with the tips of their necks protruding
from the stroma; C, an ascus with 8 ascospores; D, two ascospores. (Drawing
by Lenore Gray).
|
Back to Top
Control
A. General Control Measures
- Grow varieties of poplars and willows that are well adapted to the area
and planting site. Select only vigorous, disease-free nursery stock. Avoid
planting susceptible varieties such as Lombardy, Simon and Siouxland poplars.
Instead, grow one of the resistant varieties now available. Black and peach
willows are reported as being resistant.
- Remove all dead and dying branches on affected trees. If cankers are confined
to twigs or branches, diseased bark and discolored wood may be removed with
a sharp knife by cutting back 1 to 2 inches into surrounding live, healthy
tissues. Whenever possible, the wound should be shaped into a vertical oval
or ellipse with rounded ends. Avoid leaving branch stubs. Do not prune or
work around trees when the bark is wet as this helps to spread the fungus.
Pruning tools should be sterilized between cuts by swabbing them with 70 percent
rubbing alcohol or fresh household liquid bleach (1 part of bleach to 9 parts
of water). Remove and burn or bury all affected parts as soon as possible.
Severely cankered trees cannot be restored to good health and should be cut
down and burned because they are a source of infection for other trees.
- Some trunk cankers, if less than halfway around the stem, can be successfully
removed by careful surgery of all diseased bark and the underlying discolored
wood. This work is best done by a licensed and experienced arborist.
- Treat all bark and wood injuries promptly. Cut away all loose or discolored
bark. Clean, smooth, and shape the wood into an oval or ellipse with rounded
tips and its long axis oriented vertically. Swab the wound surface liberally
with shellac or 70 percent alchohol. Many arborists then coat the wound with
a tree wound dressing or paint. The use of commercial tree paints is not generally
recommended as their effect is largely cosmetic. Surgery may prolong the lives
of some severely affected trees.
- Keep plants growing vigorously by (a) proper applications of a balanced
fertilizer in mid to late autumn or early spring based on a soil test; (b)
watering deeply (soil moist 10 to 12 inches deep) during hot, dry weather
(repeat at 10-day intervals as long as the drought continues); (c) proper
pruning; and (d) winter protection of young tree trunks using strips of burlap
or special tree wrapping paper to prevent sunscald and bark injury.
- Avoid all unnecessary bark wounds. Keep the trunk base as dry as possible
and free of grass, weeds, or other debris that might attract rodents.
- Avoid chemical injuries. Apply herbicides and other pesticides, salt, fertilizers,
and other chemicals strictly according to label directions.
- No chemical treatment has been shown to prevent or arrest the development
of cytospora canker on poplars and willows.
B. Disease Prevention in Nurseries and in Storage
- Cytospora canker is common in cottonwood propagation blocks in nurseries.
The disease appears to increase with the age of the blocks. It is suggested
that propagation blocks not be used for more than a 4- or 5-year period2.
- All infected nursery stock, cuttings, and propagation material should be
destroyed by burning to avoid introduction of the disease through commercial
channels.
- Precautions should be taken to prevent excessive moisture loss in nursery
material during storage. Select scion wood of high moisture content.
- Storage temperatures should be maintained above freezing and as close to
35 F (1 C) as possible with high humidity (95 to 98 percent) but without water
forming on plant material and the walls, ceiling, or floor of the storage
area.
Many factors affect seed quality. Seedborne fungi, insect damage, adverse weather
(such as frost), improper storage, and physiological aging all reduce seed vigor
and viability. Any rough or excessive handling of dry or moist seeds at harvest
or planting can cause cracked seedcoats and kill seed embryos. These cracks
may be microscopic, but they still can increase seed rot by allowing nutrients
to escape and by providing an entry for soil-inhabiting and/or seed-rotting
fungi.
Pod and stem blight and seed decay, both caused by fungi of the Diaporthe/Phomopsis
complex, are the major problems of soybean seed grown in Illinois and elsewhere
in the Midwest (Figure 2). Decayed seeds are elongated, shriveled, discolored,
and often covered with white mold growth (Figure 3). Healthy appearing, symptomless
seeds also may be infected and can develop into blighted or infected seedlings.
Seed decay is most severe when the crop has matured under high rainfall and
humidity and when harvest has been delayed by wet weather. Seedlots with 20
to 40 percent of the seeds decayed by Phomopsis spp. are not uncommon in years
when weather has favored an epidemic.
Back to Top
For further information concerning
diseases of crucifers and other vegetables, contact Mohammad Babadoost, Extension
Specialist in Fruit and Vegetable Diseases, Department of Crop Sciences, University
of Illinois at Urbana-Champaign. University
of Illinois Extension provides equal opportunities in programs and employment.
|



